Your browser is no longer supported. Please upgrade your browser to improve your experience.
We offer expert software development teams and consultants for regulated industries, such as medical, aerospace, and automotive. Our teams work closely with you to deliver the highest quality software that meets regulatory standards.
High-quality software is vital for sectors that make safety-critical products. It reduces risks and can improve product success and user satisfaction.
Our software development teams use techniques that improve quality and create fast learning cycles. This also brings more flexibility to a project when your priorities change, reassuring you that you are building the right thing.
With extensive experience working in regulated sectors, we can help at all points of the product lifecycle, from new projects to legacy software. If you have existing internal development teams and processes, our teams can integrate with them.
Our experience includes working on Software as a Medical Device (SaMD) products such as anticoagulation clinical decision support software and AI-based sample diagnostics, as well as medical devices such as a glucose meter, IVF modular workbench, and a therapeutic product for phantom limb pain. We’ve also used our Lean-Agile approach to help products, such as this diabetes self-monitoring device, get FDA compliance.
With quality being so crucial to regulated products, Lean-Agile development can give you the edge. It creates regular versions of working software and drives continuous improvement. This means:
With over 12 years of Agile experience, we are confident that it gives excellent results and improves customer outcomes. However, we can work with you even if you use Waterfall.
For medical device software or software as a medical device (SaMD), we follow IEC 62304, IEC 82304, and TIR-45. Read more about our medical software experience.
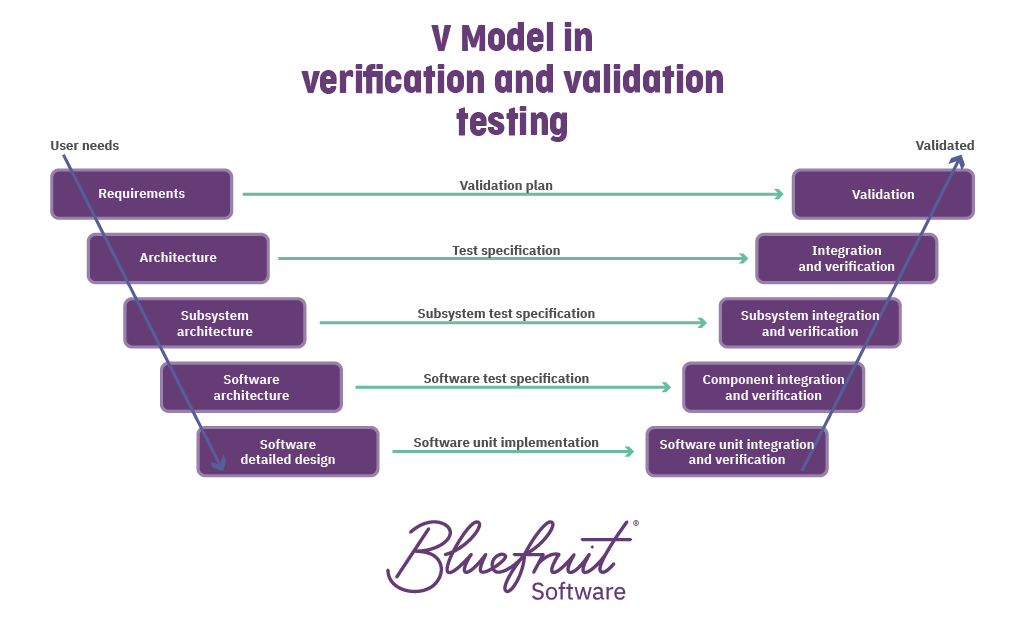
Verification and validation (V&V) of software requirements is essential to getting FDA or EU/CE approval. Our bespoke V&V teams include quality analysts and testers skilled in verification analysis and regulatory testing for medical devices.
We can work alongside your own product development teams and support you in understanding and meeting the relevant standards. Thinking about V&V during development gives the benefits of fast feedback loops that improve specifications and processes and reduces the time V&V will take at the end of a project. Alternatively, we can help with already completed products.
Whereas development teams might focus on a single feature or area, V&V teams become experts in your project requirements and constantly reflect on the broader picture. This knowledge has helped us drive improvements to your requirements, processes, software, and hardware.
Our consultants have experience working in regulated fields. Our consultancy services include the following:
Our software development teams can align with the standards required for your sector.
We have worked with classes A to C on previous medical device software and SamD projects, using IEC 62304 and FDA requirements 21 CFR Part 11, 21 CFR Part 820, and CE requirements. We can also work with ISO 13485, ISO 14971, and IEC 62366.
Our aerospace experience includes projects that meet the DO-178B standard.
Bluefruit Software has been providing high-quality embedded software engineering and testing services for more than 22 years. Our team of experienced engineers, testers and analysts has worked with a diverse range of clients and industries, including medical, scientific instruments, aerospace, automotive, consumer and more.
We can help you with software development at any project stage, ensuring quality, reliability, and security. Contact us today to discuss your software development needs.